Technology
Technology behind CORAT
CORAT antibodies are generated by the Nobel-prize awarded molecular biology technique antibody phage display to isolate human monoclonal antibodies from blood that are indistinguishable from our own body antibodies – with one difference: they prevent SARS-CoV-2 infection.
The virus binding regions of CORAT antibodies are encoded by unmodified natural human gene sequences. Each of our product candidates recognises different SARS-CoV-2 epitopes and the products are being developed either as stand-alone treatments or as combination therapy.
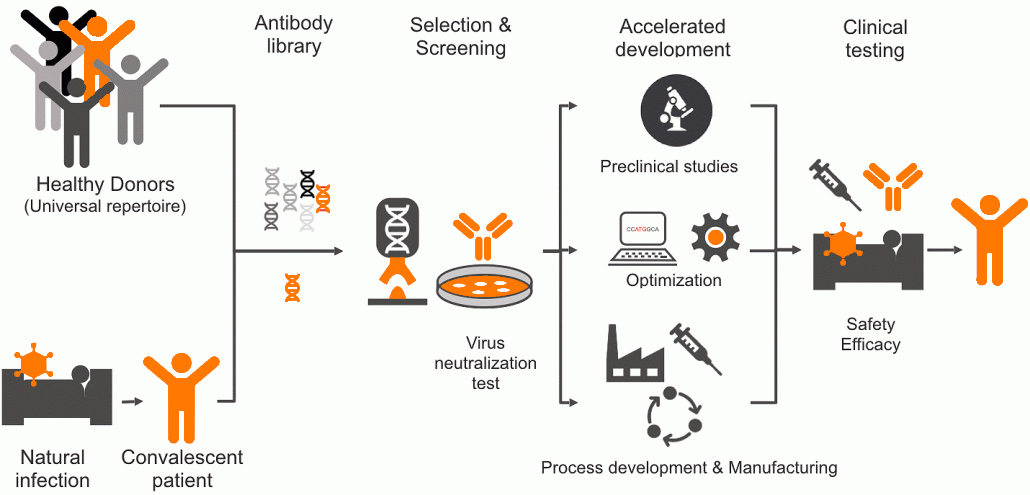
Before a final SARS-CoV-2 neutralizing antibody candidate is selected, more than 700 unique human monoclonal antibodies recognizing the SARS-CoV-2 spike protein are isolated from human antibody libraries. These libraries include antibodies from healthy donors as well as convalescent COVID-19 patients. During the selection process, a large set of anti-SARS-CoV-2 candidates undergo a fast-track screening process to identify a lead molecule with optimal drug properties.
The original gene sequences of the Fc regions of our antibodies are slightly altered with so called »silencing« modifications. These alterations prevent the binding of our antibodies to cells of the human immune system. This means that the immune system, which is already highly active after an infection, is not additionally triggered by the antibody. This unique safety feature was introduced to prevent excessive immune reactions, also known as ADE (antibody dependant enhancement), which has been described for monoclonal antibodies with an intact Fc part.
How CORAT antibodies protect against severe COVID-19
Recombinant human virus-neutralizing antibodies are the active component of immunotherapy (also named passive vaccination). Antibodies (immunoglobulins, e.g. IgGs) are molecules made by our own body to protect us against infections.
CORAT antibodies offer exactly the same protection as the body´s own antibodies that are formed due to an infection or after a vaccination. By binding to different surface molecules of SARS-CoV-2, the virus cannot invade human cells anymore. This neutralization occurs immediately after injection.
All neutralizing antibody candidates of our product pipeline are expected to be very well tolerated as they are fully human proteins. They specifically bind to SARS-CoV-2 and not to any human epitopes. Furthermore, all our antibodies are equipped with a dedicated silencing modification. This safety feature enables their use for patients with a highly active immune system that have already progressed to moderate or severe COVID-19.
How do CORAT antibodies differ from a typical vaccine?
A typical vaccine (»active vaccine«) consisting of dead or partial virus material or genes encoding virus materials cannot cure COVID-19 patients. The reason for this is that active vaccines induce an immune response – usually the generation of antibodies (IgG) – just as the infection does itself. However, the development of protective antibodies takes up to 2-3 weeks, which is too long especially for patients with severe COVID-19. Patients who are already infected therefore do not benefit from such an active vaccine. The same applies to medical care personnel in emergency situations, as immunological protection sets in only several weeks after vaccination.
In contrast, CORAT antibody immunotherapy (also named »passive vaccine«) supplements the antibodies that are either not yet or insufficiently present in the patient’s body. They help to lower the virus burden almost immediately after administration.
COVID-19 vaccines have made an invaluable contribution to pandemic containment. Nevertheless, the development of COVID-19 therapies remains essential. An active vaccine can protect healthy persons after some weeks, but it does not help patients who are already infected. It can neither provide immediate protection to risk groups and exposed medical care personnel. CORAT antibodies can fill this treatment gap and thus perfectly complement virus-based vaccines.
Is passive vaccination safe?
Serum therapy is established since 120 years and well tolerated
• Serum therapy uses (un-defined) mixtures of antibodies from immunized animals or convalescent patients to neutralize pathogens
• Introduced by Emil v. Behring in the late 19th century (First Nobel prize for medicine 1901), countless children cured (diphtheria)
• Although effective, side effects of animal serum therapy were seen, which are completely avoided by using a fully human design – the basis for CORAT antibodies
• IVIG (intravenous immunoglobulin therapy with pooled human IgG from plasma donors) is broadly used
Recombinant neutralising antibodies are a proven class of drugs
There is already an approved antibody neutralizing drug against lung viruses, which eliminates the disadvantages of serum therapy, and shows how well antibodies can be tolerated. Synagis® (palivizumab): Prevention of respiratory syncytial virus (RSV) infection, approved to be used as a precautionary measure in premature babies, infants and toddlers with previous illnesses (prophylactic administration).
Also the safety and efficacy of first neutralizing antibodies against SARS-CoV-2 has been demonstrated. Regulatory bodies such as the European Medicines Agency (EMA) or the US FDA advice on the use of different therapeutic antibodies to treat mild or moderate COVID-19. However, so far all programs have failed to show benefit in the treatment of severe COVID-19.
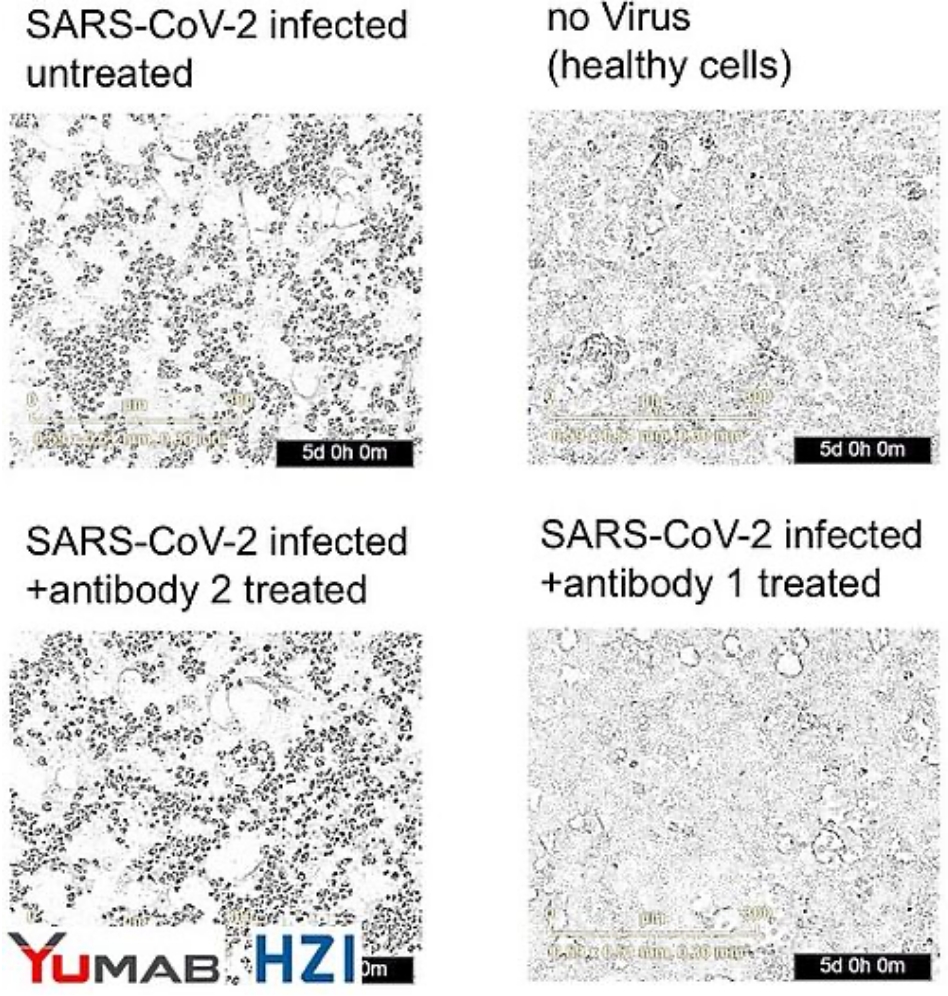
CORAT antibody prevents infection with SARS-CoV-2 isolated from COVID-19 patient in vitro
In cell culture, infection of human lung cells with SARS-CoV-2 isolates from a COVID-19 patient lead to their lysis, as indicated by rounding and loss of confluence (upper left panel). Uninfected cells (upper right) grew normally (no dead round dots). Lower right: Adding a CORAT antibody to infected cells completely protected the cells from infection for 5 days, while a negative control antibody (lower left panel) did not protect the cells.
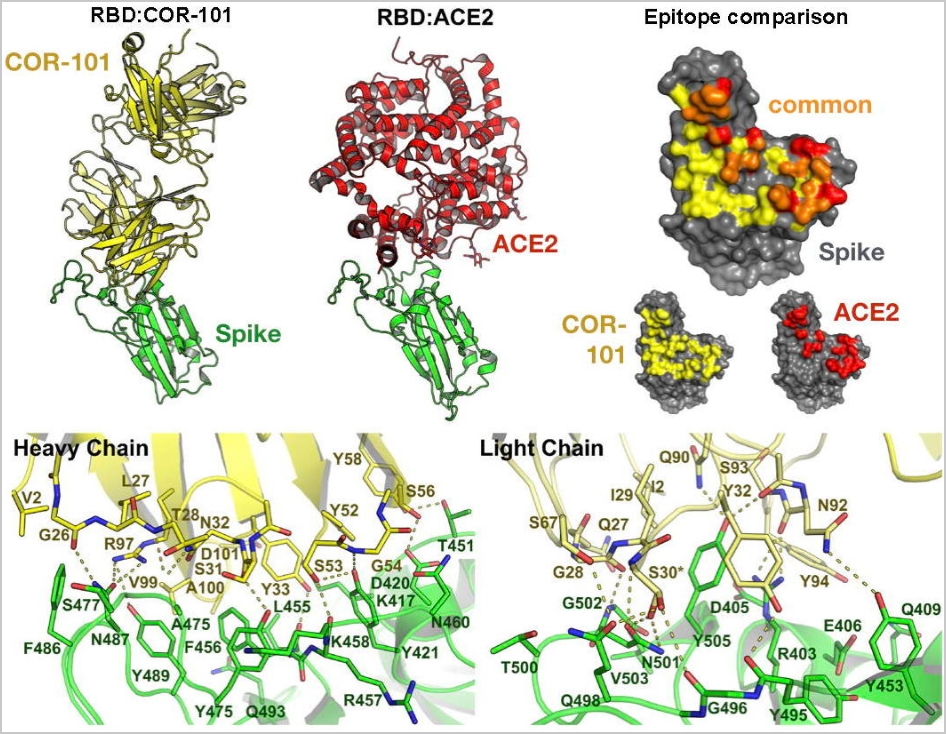
How the human antibody COR-101 neutralizes SARS-CoV-2
The crystal structure of COR-101 (yellow) Fab in complex with SARS-CoV-2-RBD (green). Bertoglio et al, Cell Rep. 2021, 36(4):109433.
The fully human antibody COR-101 blocks SARS-CoV-2 by inhibition of ACE2 binding and prevents infection with the coronavirus.
COR-101 showed an IC50 of 0.56 nM in a plaque-based live SARS-CoV-2 neutralization assay. The crystal structure of COR-101 in complex with SARS-CoV-2-RBD was solved at 2.0 A resolution showing that the antibody binds at the same region as the human coronavirus receptor ACE2 to SARS-CoV-2-RBD (see also here). COR-101 covers an extraordinarily large area and with very high binding strength. As a result, the virus can no longer attack and penetrate the cells and multiply.
Bertoglio et al. »A SARS-CoV-2 neutralizing antibody selected from COVID-19 patients by phage display is binding to the ACE2-RBD interface and is tolerant to known RBD mutations.«, Cell Rep. 2021 36(4):109433.
https://doi.org/10.1016/j.celrep.2021.109433